A Bravo Probe in Children
The Bravo Probe in Children article is about Upper Esophageal pH
monitoring of Children with the Bravo pH capsule. It is written by
Marcella Bothwell, MD; Jeffrey Phillips, Pharm D and Susan Bauer, APN.
The Laryngoscope, Lippincott Williams & Wilkins, Inc.© 2004. The American Larygological, Rhinological and Otological.
Introduction & Explanation of Placing Bravo Probe in Children
Reflux of gastric contents is a common, well documented occurrence among adults. Since the early 1980s, gastroesophageal reflux disease (GERD) has been increasingly recognized as a clinical entity among children. Several investigators have noted that approximately half of all newborns have reflux. In the study of Nelson et al., peak incidence of reflux occurred at approximately 4 months of age; approximately 67% of these infants exhibited signs of reflux disease. Prevalence decreased to approximately 5% at 1 year of age.
Left untreated, gastroesophageal reflux most commonly results in vomiting (spitting up), abdominal or chest pain, heartburn, arousal from sleep, and regurgitation. Studies have found that up to 75% of patients with symptoms of upper or lower respiratory tract disorders such as asthma, croup, bronchitis, pneumonia, sinusitis, laryngomalacia, and subglottic stenosis have reflux disease. Gastroesophageal reflux may also be associated with more serious complications such as apparent life threatening events, isolated bradycardia with irregular respiratory efforts, apnea, hypotension, reduced cerebral blood flow, and sudden infant death syndrome (SIDS).
Although reflux is a common occurrence among young children, making a confirmatory diagnosis is difficult in the clinical setting. Pediatric patients are not always cooperative with a 24 hour pH study. Questions about the validity of such studies exist, and the interpretation of the findings can be difficult.
Technique of placing a Bravo Probe in children
Children suspected of having gastroesophageal reflux affecting the upper
airway have a pH probe or “capsule” put into place in the operating
room while undergoing other procedures (predominantly, direct
laryngoscopy with bronchoscopy) If there are no plans for surgery, the
traditional pH probe is placed through the nose by our colleague. The
Bravo capsule technique is used to enhance the diagnosis of reflux, ot
to completely change how we diagnose reflux.
The delivery system
and capsule are manufactured by Medtronic (reference no. 9012B1001,
Shoreview, MN). The delivery system is 100 cm long, and the size of the
capsule is 26 6.06.3 mm (Fig. 1).
 pH probe in the esophagus of an infant with acid reflux
pH probe in the esophagus of an infant with acid refluxFor an otolaryngologist trained in upper airway management, this
technique has been uncomplicated. After securing intubation with an
appropriately sized endotracheal tube, the Parsons laryngoscope is
reinserted, lifting the larynx forward. This identifies the
cricopharyngeal opening to the esophagus. The capsule is placed in the
proper position, and the suction device is attached. A small amount of
mucosa is gathered and pinned by the delivery device. The capsule is
released from the delivery system and remains in place. When detaching
the capsule from the delivery system, it is important to turn off the
suction machine and also disconnect the suction device from the delivery
vehicle to remove any residual suction from the system. This allows a
much easier release of the capsule onto the esophageal mucosa without
injury. This also aids in the prevention of capsule related
complications. Esophagoscopy has been routinely used to confirm
appropriate placement in the upper esophagus, but it is not necessary.
Although
it would be ideal to have pH measurements from the posterior pharynx to
diagnose extraesophageal reflux, this capsule should rest just below
the closed cricopharyngeal muscle in the upper esophagus. The
cricopharyngeal muscle is composed of striated muscle and is tonically
contracted to protect the airway from refluxed esophageal contents
(Figs. 2 and 3).
While the capsule is in place, the child wears a
receiver device, which is approximately the size of a pager. The
receiver need not be worn but should remain within 3 feet of the child
to ensure proper recording of the data. The child can go home and is
allowed to eat normally. No changes in dietary habits are necessary with
the capsule. Some procedures require children to be on restricted diet
regimens, and this could potentially confound pH data with this
particular device.
The capsule is designed to slough off the
mucosa in 3 to 5 days as the mucosa rejuvenates itself. The capsule
travels the alimentary canal and is shed in the stool a few days later.
Parents are instructed not to retrieve the capsule from the stool
because there is no need to do so.
Medtronic has a software
package that provides traditional pH measurements and is adjustable. All
data must be interpreted within the context of the individual patient
and procedure. We have found that a 48 hour time period provides us with
reliable results, and parents do not need to replace batteries (Fig.
4). As an alternative, two separate capsules can be placed at different
locations, providing 24 hours of data from each site. The reports have
been easy to interpret. With the improved data acquisition afforded by
the Bravo pH capsule, the significance of the amount and duration of
acid exposure can be more adequately investigated for the upper airway.
Case Reports of Bravo Probe in Children
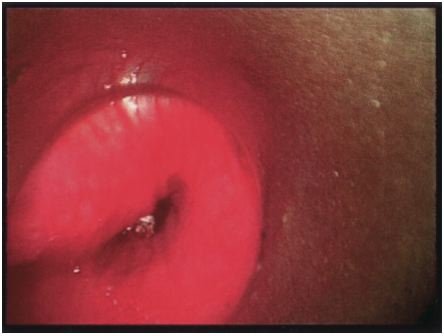 cricopharygeal sphincter covering pH capsule
cricopharygeal sphincter covering pH capsuleTwenty five children have undergone Bravo pH capsule placement. These
children were under general anesthesia for various procedures, including
direct laryngoscopy or bronchoscopy or both, tonsillectomy or
adenoidectomy or both, and sinus surgery. Age at pH capsule placement
has varied from 3 months to 11 years, depending on need, with an average
age of 3 years at the time of placement. Indications for the operating
room were various, including vomiting and dysphagia, “noisy breathing,”
chronic cough, aspiration pneumonia, reactive airway disease recurrent
croup, known subglottic stenosis, nasal airway obstruction with
rhinosinusitis, sleep apnea, and restrictive lung disease. Three
children have had lansoprazole administered at the approximate 24 hour
mark, and pH measurements markedly improved rapidly (Fig. 4). We have
observed no serious complications as a result of this test. In only one
patient was the capsule difficult to remove from the delivery system; a
small tear in the superficial mucosa resulted. The child was observed
overnight for complications of mediastinitis, but none was noted. No
other children from the study group encountered complications as a
result of the evacuation of the capsule.
 48hr pH tracing in infant with acid reflux
48hr pH tracing in infant with acid refluxDiscussion Of The Bravo Probe In Children
At the University of Missouri (Columbia, MO), we have successfully placed 25 Medtronic Bravo pH capsules. After discovering that the suction device should not only be turned off but also disconnected from the delivery system, we have had no capsule related complications. It is important for those not familiar with the complications of the “airway foreign body” to be cognizant of placing the capsule just below the protection of the cricopharyngeal muscle sphincter.
The mere diagnosis of extraesophageal reflux has been controversial, including such features as the level of pH, time of acid exposure, and number of incidences. Patients with extraesophageal reflux may have a negative finding on esophagoscopy for reflux. However, extraesophageal reflux can cause considerable damage to the tender respiratory epithelium of the larynx, trachea, nose, and middle ear. Pharyngeal probes have been instrumental in the diagnosis of a disease that otherwise was scoffed at by the community of gastroenterologists. A negative finding on proximal probe does not exclude the diagnosis of reflux into the pharynx. The Bravo pH probe is used
in addition to traditional reflux tests. It can produce useful information related to treatment (Fig. 1).
Some children, as well as some parents, are reluctant to place conventional pH probes because they appear to be uncomfortable. Indeed, the conventional probe tubing may produce artifactual reflux episodes. By having the 48 hour period to study the child, we are able to study the effects of lansoprazole at 24 hours into the data gathering period. Clearly, the “normalization” of pH during medical management is evident from early data.
Children at our institution have done exceedingly well using the Bravo pH capsule system. We recommend it for its ease of placement, its comfort to the patient, and the duration of the data obtained. Studies for advanced methods of interpretation and understanding of the retrieved pH data are under way.
The Bravo Probe In Children PDF Download
- MatthewsB,LitleJ,McGuirtWF,KoufmanJ.Refluxininfants with laryngomalacia: results of 24-hour probe pH monitoring. Otolaryngol Head Neck Surg 1999;120:860–864.
- Nelson S, Chen E, Syniar G, Christoffel K. Prevalence of symptoms of gastroesophageal reflux during infancy. Arch Pediatr Adolesc Med 1997;151:569–572.
- Andze G, Brandt M, St Vil D, Bensoussan A, Blanchard H. Diagnosis and treatment of gastroesophageal reflux in 500 children with respiratory symptoms: the value of pH monitoring. J Pediatr Surg 1991;26:295–300.
- Waki E, Madgy D, Belenky W, Gower V. The incidence of gastroesophageal reflux in recurrent croup. Int J Pediatr Otorhinolaryngol 1995;32:223–232.
- Giannoni C, Sulek M, Friedman E, Duncan N. Gastroesophageal reflux association with laryngomalacia: a prospective study. Int J Pediatr Otorhinolaryngol 1998;43:11–20.
- Contencin P, Narcy P. Nasopharyngeal pH monitoring in infants and children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 1991;22:249–256.
- Walner D, Stern Y, Gerber M, Rudolph C, Baldwin C, Cotton R. Gastroesophageal reflux in patients with subglottic stenosis. Arch Otolaryngol Head Neck Surg 1998;124: 551–555.
- Gray C, Davies F, Molyneux E. Apparent life threatening events presenting to a pediatric emergency department. Pediatr Emerg Care 1999;15:195–199.
- Bothwell MR, Parsons D, Talbot A, Barbaro GJ, Wilder B. Outcome of reflux therapy on pediatric chronic sinusitis. Otolaryngol Head Neck Surg 1999;121:255–262.
- Little J, Matthews B, Glock M, et al. Extraesophageal pediatric reflux: 24-hour double probe pH monitoring of 222 children. Ann Otol Rhinol Laryngol 1997; 106(Suppl 169): 1–16.
In addition, the last sentence of the second paragraph of the discussion should read, “In the present report, both histological and biomechanical shear strength properties of the bone periosteum interface healing under tension were studied.”